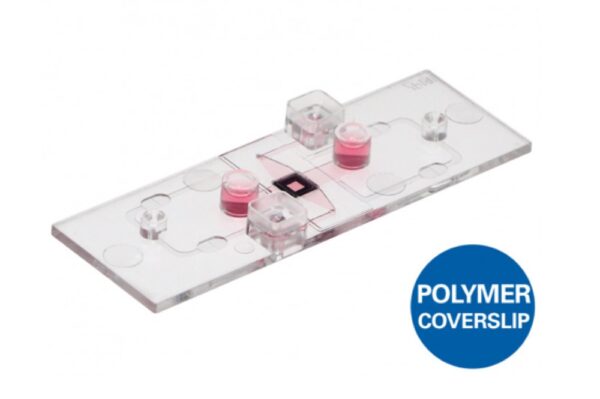
Ibidi – Labware – µ-Slide Membrane ibiPore Flow
Product Outline:
- A µ-Slide with a porous glass membrane for transmigration and transport studies under both static and flow conditions
- Brilliant optical quality through the thin, porous glass membrane
- Full access to the apical and basal sides of adherent cells in numerous applications
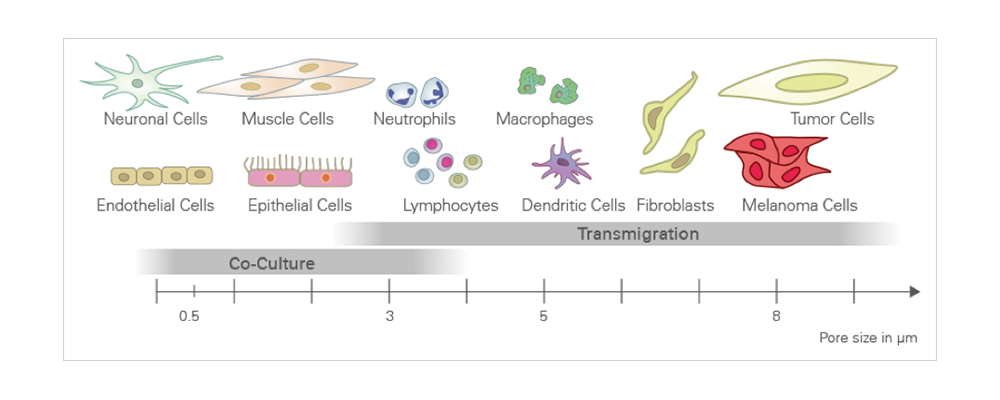
- Different pore sizes available for various cell types
- Suitable for the establishment of lung models with air-liquid interface (ALI)
Applications
- Trans-endothelial migration under flow conditions
- Co-cultivation of cell layers and transport in 2D or in a 3D gel matrix
- Apical-basal cell polarity assays
- Skin and lung models with liquid-air interface
- Cell barrier model assays with apical-basal gradients
- Cell migration assays based on filters and porous membranes
Technical Specifications
| Total coating area | 4.50 cm² |
| Bottom | ibidi Polymer Coverslip |
| Main Channel (Lower Channel) | |
| Access | Luer port, accessible with female Luers |
| Volume | 50 µl |
| Height x Length x Width | 0.4 mm x 25 mm x 5 mm |
| Growth area | 1.25 cm² |
| Upper Channel | |
| Access | Reservoir port, accessible with 20/200 µl pipet tips |
| Volume | 55 µl |
| Height over membrane | 1.3 mm |
| ibiPore Membrane | |
| Material | SiO2 (glass) |
| Thickness | 0.3 µm (300 nm) |
| Membrane size | 2 mm x 2 mm |
| Porous area | 1.77 mm x 1.84 mm |
| Restrictions for objective lenses Working distance >0.5 mm | Restrictions for objective lenses Working distance >0.5 mm |
| Pore layout | Hexagonal spacing |
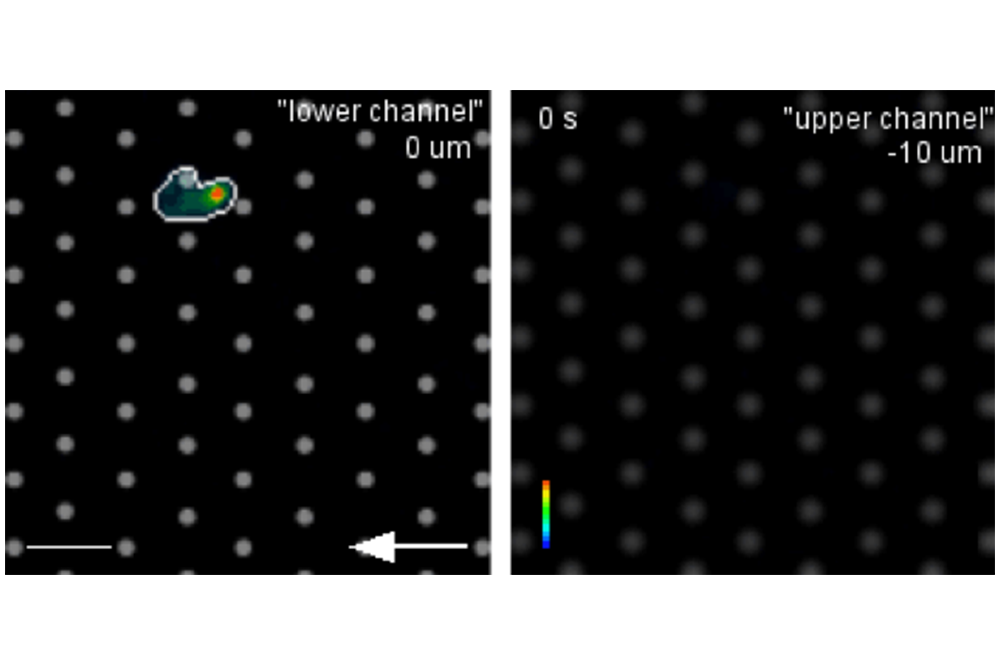
Transmigration Under Flow
Shown here is a time lapse movie of a transmigrating human HL-60 cell that was seeded into the lower channel of a µ-Slide Membrane ibiPore Flow (pore size 3 µm). Under flow conditions, the cell transmigrates in vitro through the porous glass membrane into the upper channel that was filled with a 3D collagen matrix containing the chemoattractant fMLP.
Live cell imaging of a transmigrating single SiR-Actin-stained cell (indicated by pseudo colors) by spinning disc confocal microscopy (20x objective) with a time interval of 30 s. The arrow indicates the direction of flow at a shear stress of 1 dyne/cm2; Scale bar = 10 um. Courtesy R. Pick, Munich, Germany.
Technical Features
- SiMPore’s G-FLAT™ Microporous Glass Membranes
- Cross-channel structure with a porous optical membrane in between
- Excellent optical properties comparable to a glass coverslip
- Pore sizes 0.5 µm, 3 µm, 5 µm, or 8 µm available
- Membrane thickness 0.3 µm (300 nm)
- For use with objective lenses with a working distance >0.5 mm
- Fully compatible with the ibidi Pump System
- Defined shear stress and shear rate levels
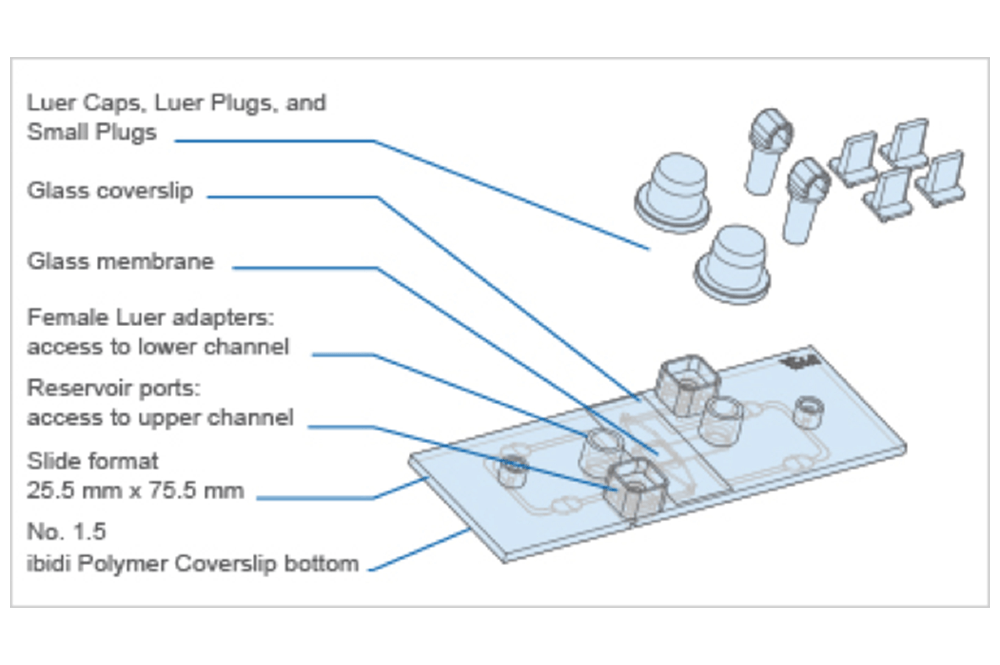
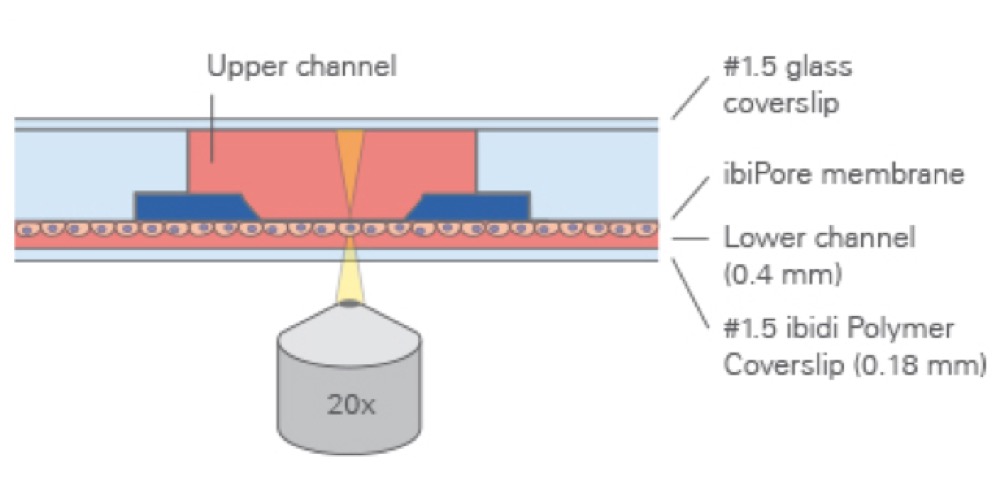
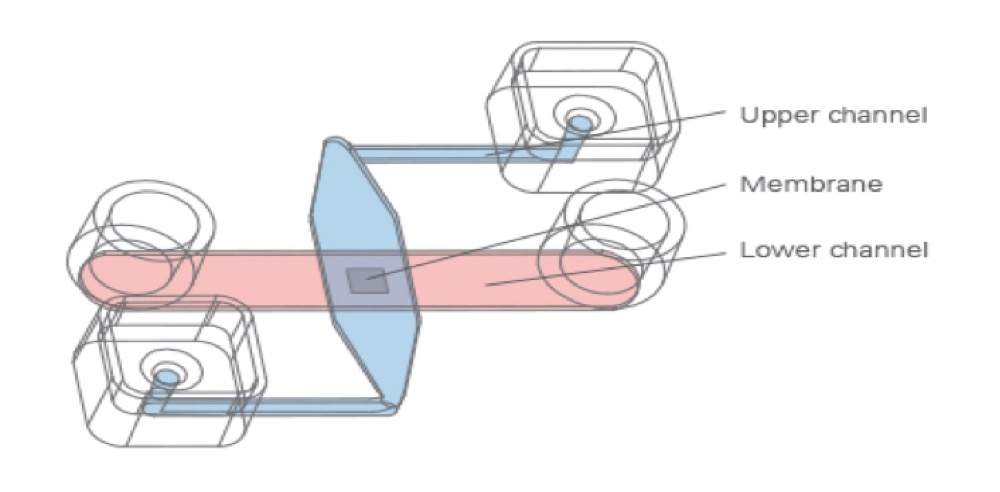
The Principle of the µ-Slide Membrane ibiPore Flow
The μ-Slide Membrane ibiPore Flow consists of a horizontal porous glass membrane that is inserted between two channels. The upper channel is a static reservoir above the membrane. The lower channel is a perfusion channel for applying defined shear stress on cells, which are attached to the membrane. The upper and the lower channel communicate with each other only across the membrane.
What Is the Porosity?
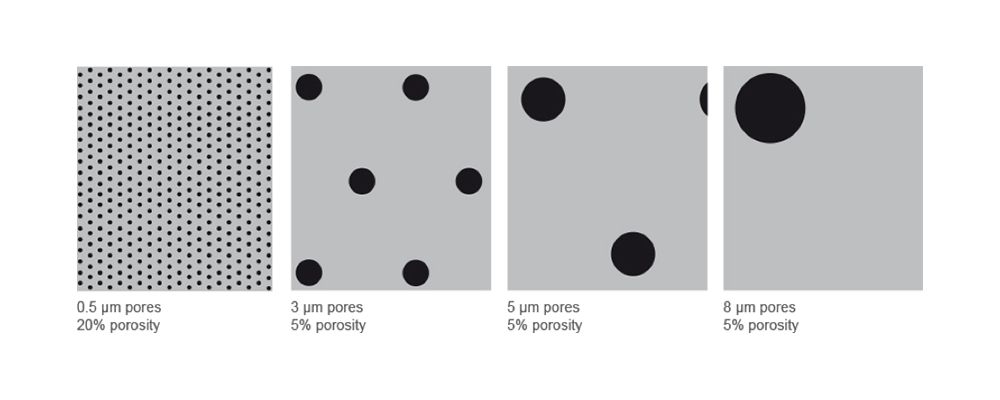
Porosity refers to the void volume fraction of the membrane. It is defined as the volume of the pores divided by the total volume of the membrane.
Technical Specifications
| Membrane Pore Size and Porosity | Applications | Example Cell Types |
|---|---|---|
| 0.5 µm pores, high porosity (20%) | Permeability and transport studies, co-culture models | Lung cells, epithelial cells |
| 3.0 µm pores, low porosity (5%) | Transendothelial migration | Leukocytes (e.g., neutrophils), lymphocytes (e.g., T cells or B cells) |
| 5.0 µm pores, low porosity (5%) | Invasion, migration | Monocytes, macrophages, lymphocytes (e.g., T cells or B cells) |
| 8.0 µm pores, low porosity (5%) | Invasion, migration | Tumour and cancer cells, endothelial and epithelial cells, fibroblasts, osteoblasts, melanoma cells, glioma cells |
The bigger the cell, the bigger the recommended pore size.
Intended Use
These applications have been tested by the ibidi R&D team or by our customers.
Endothelial Barrier Assays
A cell monolayer is cultivated on one side of the membrane.
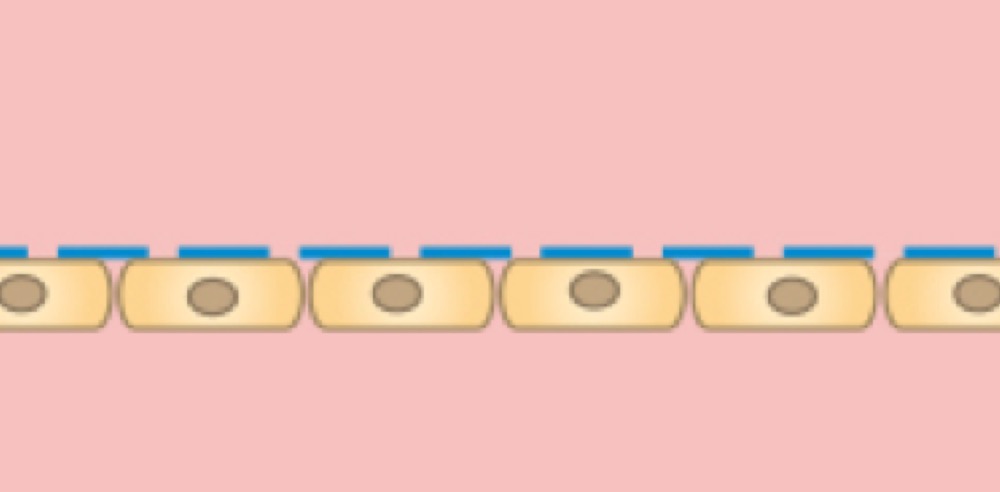
Cells on the lower side of the membrane
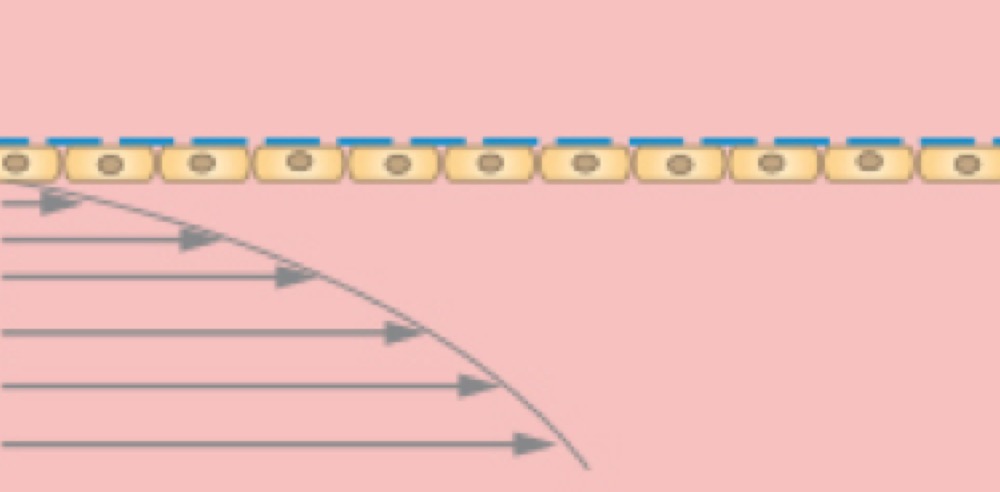
Cells on the lower side of the membrane with defined shear stress
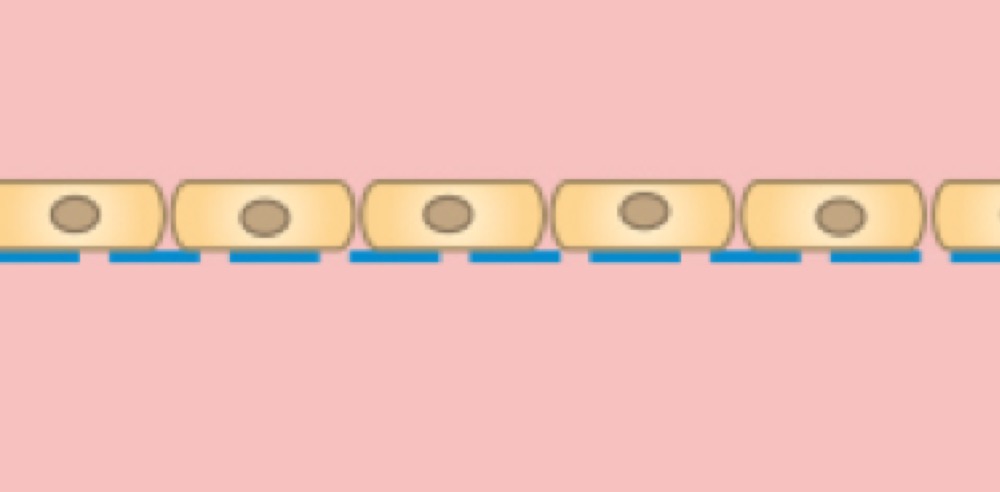
Cells on the upper side of the membrane
Co-Culture and Cell Barrier Assay
Two separate cell monolayers are cultivated on each side of the membrane. With this method, signaling, co-culture, and transport studies are possible.
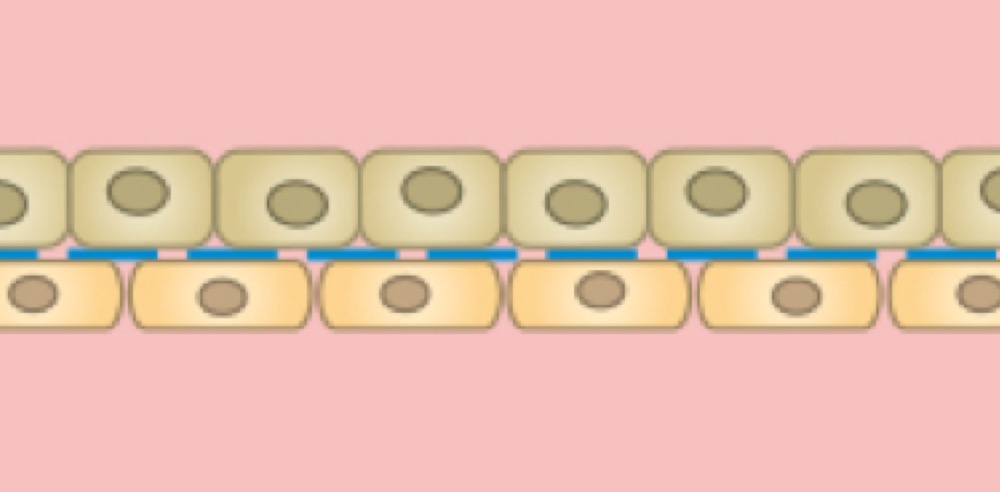
Co-culture of two different cell types on both sides of the membrane
Apical-Basal Cell Polarity Assays
Chemical factors inside of a 3D gel matrix lead to the polarization of a cell monolayer that is cultured on the other side of the membrane.
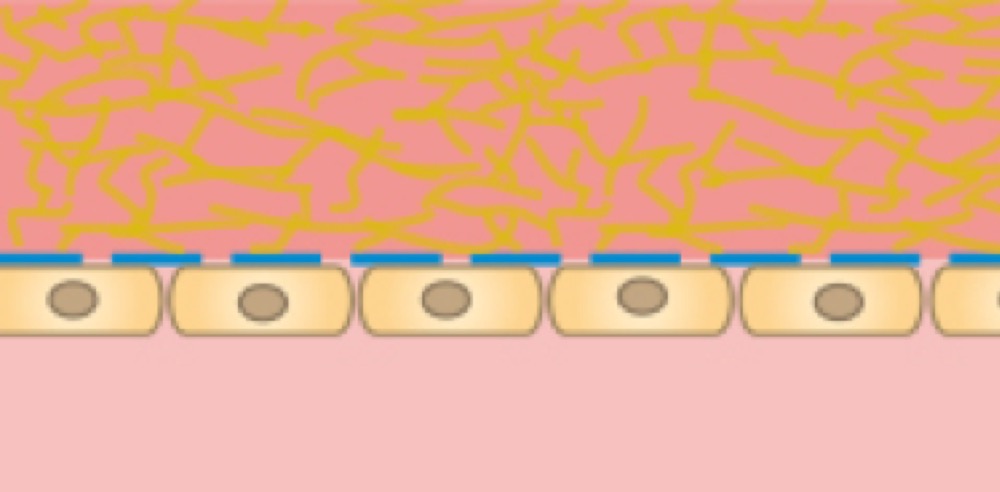
Cells on the lower side of the membrane with a 3D gel matrix in the upper channel
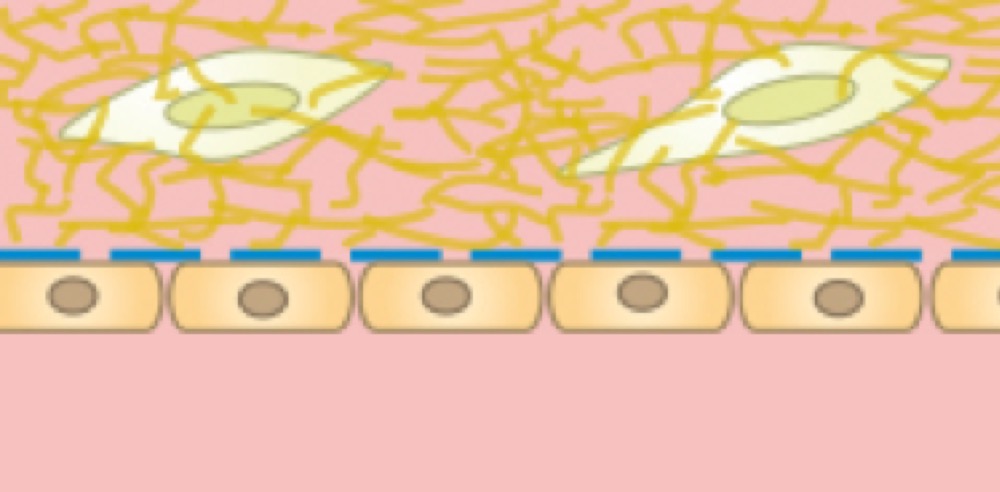
Cells on the lower side of the membrane with a 3D gel matrix with embedded cells in the upper channel
Skin and Lung Models with Air-Liquid Interface
A polarized cell monolayer (e.g., lung epithelial cells or skin cells) is cultured on one side of the membrane, which is exposed to air.
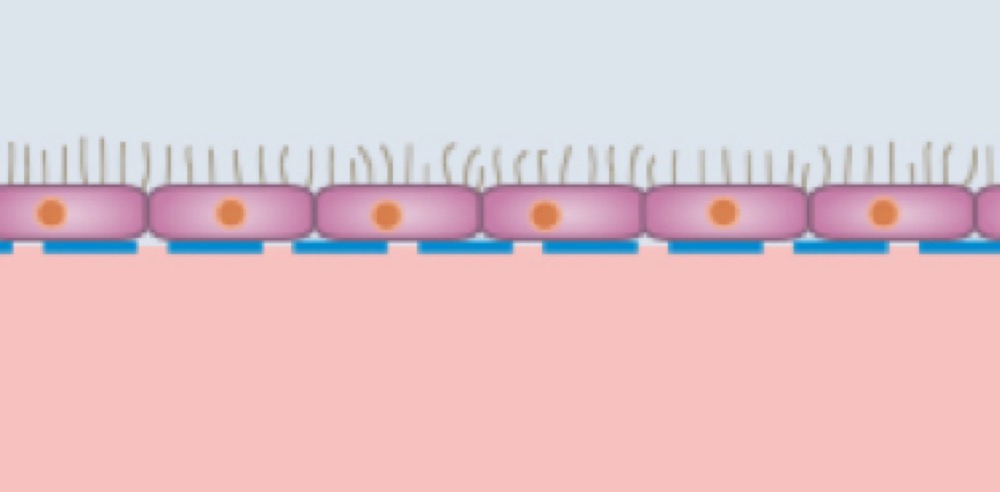
Epithelial cells, such as lung cells, on the upper side of the membrane, exposed to air in the upper channel and supplied by culture medium from the lower channel
More Products
ibidi is a leading supplier for functional cell-based assays and advanced products for cellular microscopy. ibidi is located in Gräfelfing,…